
End-to-end continuous clinical safety
We automate the hardest parts of clinical safety, connecting you with all your staff across all your organisations, continuously through each phase of the technology lifecycle.
Automated DCB0160 documentation
Our automation handles the most time-consuming parts of DCB0160, freeing your clinical leads to focus on reviewing and approving the final documents.
What we provide:
- Clinical Safety Case Reports
- Clinical Risk Management Plans
- Clinical Risk Management Systems
- Hazard Logs
- Post-Deployment Monitoring Reports
- Hazard Log Updates
- Decommissioning Reports
Engage your entire workforce
Our virtual hazard workshops turn your staff into collaborators and co-designers of safe digital change.
We trust the people in the system, not distant "experts". Real insight comes from those doing the work on the ground.
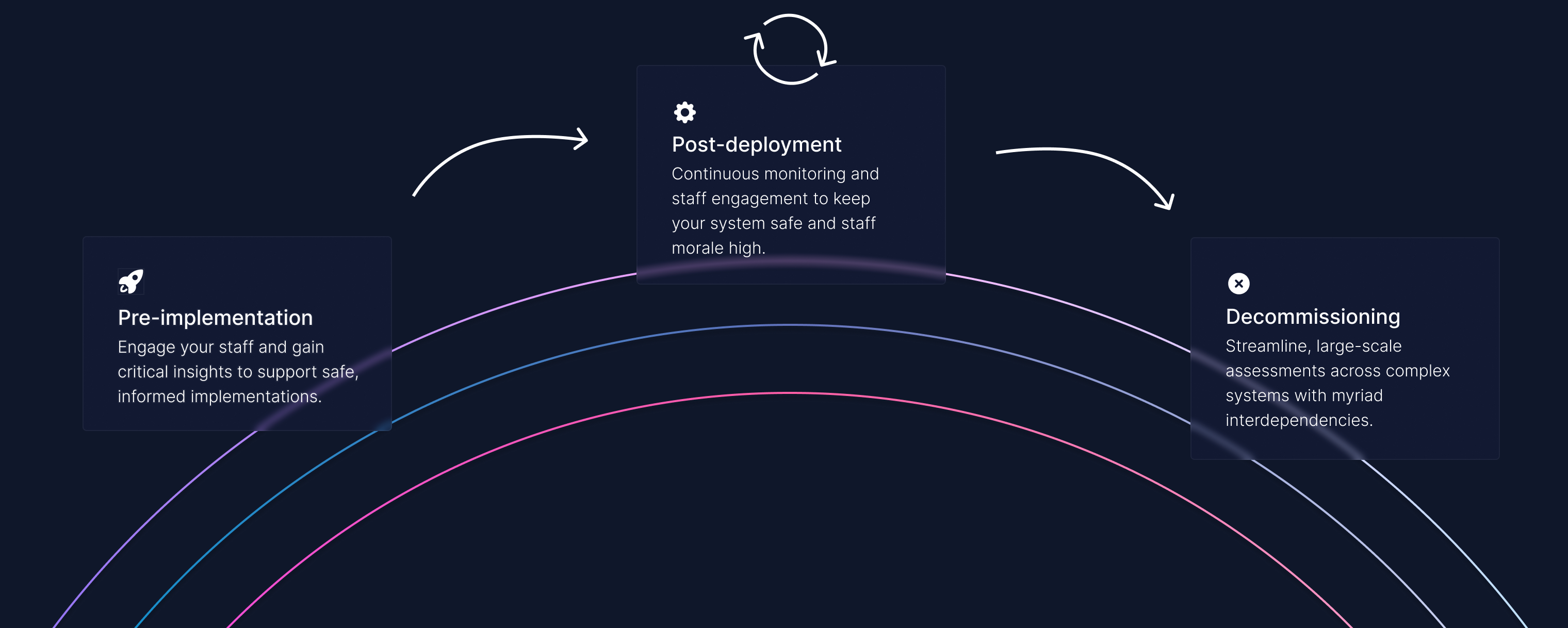
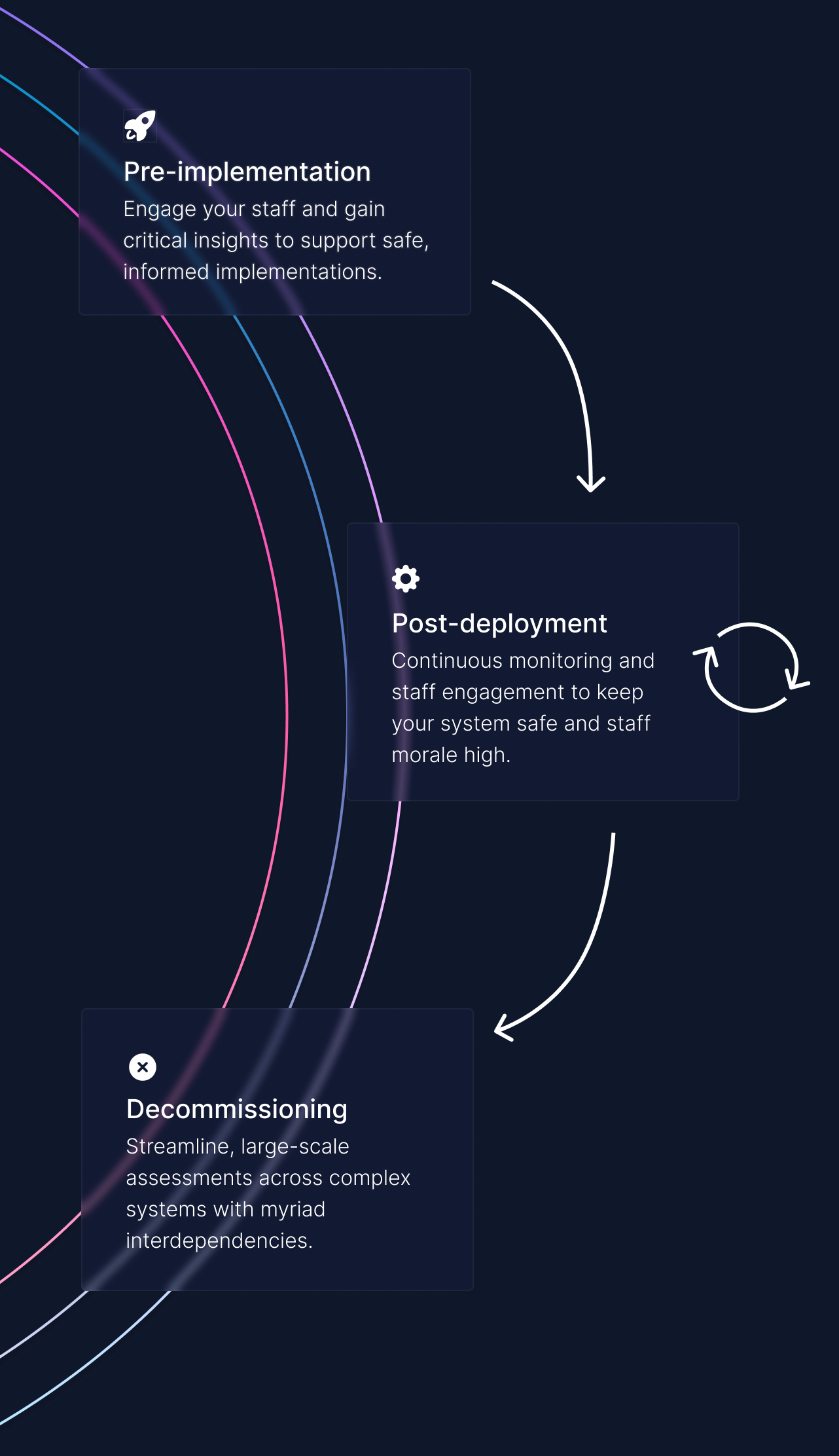
With you at every stage of the product lifecycle.
Clinical safety doesn't stop at the launch of a product. It is an ongoing process that requires continuous monitoring, feedback and improvement.
Trusted in the NHS.
We're trusted by digital safety leads in the NHS Nationwide. But don't just take our word for it — hear what our customers have to say.

DCB0160 has been a wicked problem for our Integrated Care System. Bordercross Health has supported us with multiple DCB0160 product assessments across all lifecycle phases this year.
These included a pre-implementation review of Anima Triage across GP practices in Kent, a Tortus pre-implementation across primary, community and secondary care organisations, and a major decommissioning assessment involving over 200 organisations across all sectors.
Their product enabled us to complete these assessments within weeks, significantly accelerating our timelines — and at a cost significantly lower than traditional approaches."





Having struggled with clinical safety with a number of tools for our practices across North East London, we've had some great discussions with Bordercross to put together a process for clinical safety to not only benefit our practices, but also support the approach that the ICB required for oversight and maintaining a centralised hazard log.
It has been a supportive and smooth process that not only built confidence but also enabled us to roll out clinical tools like Primary Care IT and AI scribes from providers such as Heidi Health and Accurx to our practices in a fraction of the time and cost it would have previously taken."





We partnered with Bordercross Health on two complex, multi-sector projects – an ambient voice technology pre-implementation assessment and a de-commissioning assessment for a shared record feed.
The support we received was invaluable. The platform gathered stakeholder feedback quickly, turning insights into actions. Thanks to the success of this, we are now reviewing our options for supporting compliance with the clinical safety standards on another project.

We live and breathe clinical safety.
Behind every DCB0160 and report is a real person who cares about getting it right — our team combines frontline clinical experience with deep technical expertise.
Get in touch
Whether you're ready to get started or just have a few questions, our team is here to help. Drop us a message — we'll get back to you shortly.
General enquiries
- General enquiries








